Abstract
Introduction: Extramedullary disease (EMD) is a poor prognostic feature with an increasing incidence in patients (pts) with multiple myeloma (MM). Efficacious therapies are needed, with a good safety profile which can override the negative impact. Daratumumab (an IgGκ monoclonal anti-CD38 antibody) with cyclophosphamide, bortezomib and dexamethasone (DaraVCD) is a novel combination with a good efficacy profile in EMD, based on preclinical synergistic data.
Methods: EMN19 is an ongoing, phase 2, open-label study (NCT04166565); MM pts presenting with EMD at diagnosis or following 1 line of treatment, but not refractory to bortezomib-based regimens, were recruited from 8 sites (Turkey, Greece, Italy). Pts had measurable disease (monoclonal protein and/or light chain in the serum or urine) at screening. Pts with bortezomib or daratumumab hypersensitivity, autologous stem cell transplantation (ASCT) within 12 weeks (wks) before Cycle (C)1 or previous allogenic stem cell transplant were excluded. DaraVCD is administered until progression or unacceptable toxicity for a minimum of 3 cycles to evaluate response unless refractory disease is detected by the end of C3 (progressive disease [PD] or failure to achieve a confirmed partial response [PR] or better). Daratumumab was initially administered intravenously at 16 mg/kg, and, since July 2020 subcutaneously at a fixed dose of 1800 mg, weekly during C1-2, every 2 wks for C3-6, and every 4 wks thereafter. Responses were assessed according to IMWG and Impetus criteria (Zamagni, E. et al., 2021, DOI: 10.1200/JCO.20.00386). ASCT is allowed during study treatment, as per the physician's decision. As a correlative study, circulating tumour cells (CTC) were quantified in blood for all pts at baseline (BL) by EuroFlow Next Generation Flow cytometry. After red blood cells lysis, peripheral blood was stained using the following markers: CD38, CD138, CD45, CD19, CD27, CD56, CD81, CD117, CyIgK and CyIgL. The samples were measured on a FACSCanto or FACSLyric machine; data analysis was performed in Infinicyt.
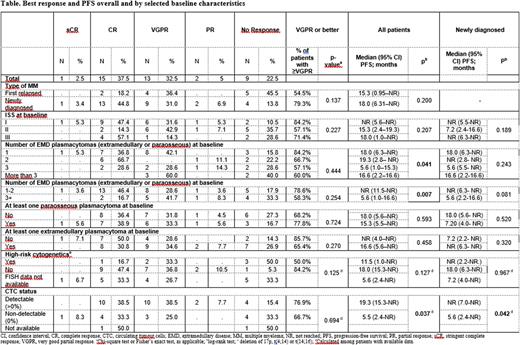
Results: 29 (72.5%) newly diagnosed (NDMM) and 11 (27.5%) first relapsed pts were enrolled (55% male; median age 58 years; range 37-77). ISS stage was I, II and III for 19 (47.5%), 14 (35%) and 7 (17.5%) pts, respectively; 6 of 25 FISH evaluable pts were high-risk (according to IMWG); 26 (65%) pts had ≥1 extramedullary (EMP) and 18 (45%) had ≥1 paraosseous (PO) plasmacytoma (4 had both). On average, 2.5 (range 1-13) plasmacytomas (PCTs) were observed per patient. 6 (15%) pts received ASCT during the study treatment period at a median of 7 months after the start of C1.
At the cut-off date (31 May 2022; median follow-up: 12 months), 23 (58%) pts were on treatment and 17 had discontinued (inadequate response after the end of C3: 3 pts; PD: 11 pts; death: 3 pts). 16 (40%) pts achieved complete response (CR; 1 of which was stringent), 13 (33%) very good PR and 2 (5%) PR. Median (range) time to first response was 4 (<1-17) wks. 3/6 pts with ASCT achieved CR prior to ASCT. Median OS was not reached (9 deaths occurred); median (95% CI) progression-free survival was 18 (7-not reached) months. NDMM, absence of EMP and lower number of PCTs were associated with better responses (Table). 16 pts had EMD response data; 14 had complete (CMR) and 2 had partial metabolic response according to Deauville score. Of those with CMR, 4 achieved CMR prior to hematologic CR, for 8 first assessment of EMD response was after CR and for 2 CR had not been achieved yet. 37 (93%) pts had at ≥1 adverse event; 14 ≥1 serious and 19 ≥1 Grade 3 or 4 TEAE.
CTC (median level: 0.002%; range 0-0.35%) were detected at intake in 26 out of 38 evaluable cases; 8 were PO only, 16 EMP only and 2 had both. CTC levels were similar between pts with EMP vs only PO. Univariate analysis of ISS, FISH, PCTs number and CTC level showed that number of EMDs at BL is associated with PFS (HR: 1.19 (1.02-1.39); p=0.03).
Conclusions: Primary objective was reached, with no safety signals and a good efficacy profile across subgroups. NDMM and fewer number of PCTs at BL were related with better and deeper responses. DaraVCD was effective regardless of the level of CTC. The preliminary results of this prospective clinical trial are in accordance with the final results of the Lyra study where CR or better was achieved with DaraVCD among 48.7% and 29.8% for NDMM transplant and non-transplant pts suggesting this regimen as an effective future treatment option for pts presenting with EMD.
Disclosures
Beksac:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gay:BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees. Mina:GSK: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Katodritou:Takeda: Honoraria, Other: research expenses, Research Funding; Abbvie: Honoraria, Other: travel expenses, Research Funding; Sanofi: Research Funding; Integris Pharma: Honoraria; Karyopharm: Research Funding; GSK: Honoraria, Other: travel expenses, Research Funding; AMGEN: Honoraria, Research Funding; JANSSEN: Other: travel expenses, Research Funding. Cavo:AbbVie, Amgen, Bristol Myers Squibb/Celgene, Pfizer, GlaxoSmithKline, Sanofi, Roche, Takeda: Consultancy, Honoraria; Janssen: Honoraria, Speakers Bureau. van der Velden:Cytognos: Other: laboratory services agreement ; Agilent: Other: laboratory services agreement ; Euroflow: Patents & Royalties; BD biosciences: Other: laboratory services agreement . Merante:EMN Italy Medical Monitor: Research Funding. Manousou:Health Data Specialists: Current Employment. Sonneveld:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Terpos:GSK: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria, Other: Travel expenses, Research Funding; Sanofi: Honoraria, Research Funding; BMS: Honoraria; Genesis: Honoraria, Research Funding; Amgen: Honoraria, Other: Travel expenses, Research Funding; EUSA Pharma: Honoraria, Other: Travel expenses; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal